Drug News
Take advantage of our Drug News page to keep abreast of current drug information. If you are looking for information that you can't find here please don't hesitate to email your suggestion/question to david@millbrookpharmacy.com.
ERGOTAMINE TOXICITY

|
GI |
Nausea, vomiting, diarrhea |
|
Fingers/Toes |
Numb, tingly |
|
Limbs |
Muscle pain, spasm, cold, tingly |
|
Cardiovascular |
Fast pulse, weak pulse, hypotension |
|
CNS |
Drowsiness, delirium, seizure, unconsciousness, death |
AFTER HEART ATTACK MEDS & TARGET DOSES

|
Class |
Med |
Target Dose |
|
ACE Inhibitors |
Lisinopril |
20mg/day |
|
Ramipril |
10mg/day |
|
|
Trandolapril |
4mg/day |
|
|
ARB |
Candesartan |
32mg/day |
|
Telmisartan |
801mg/day |
|
|
Valsartan |
320mg/day |
|
|
Beta Blockers |
Metoprolol |
200mg/day |
|
Carvedilol |
50mg/day |
|
|
Statins |
Atorvastatin |
80mg/day |
HYPOGLYCEMIA TREATMENT & PREVENTION

|
Treatment |
|
|
When |
Shaking, sweating, palpitation, dizziness |
|
Verify |
Test Blood glucose: 4 mmol/L or less (higher if elderly) |
|
|
|
|
Glucose tablets |
3-4 tablets (every 15 minutes after testing) |
|
Hard candies |
5-6 candies (every 15 minutes after testing) |
|
Regular soda |
120ml (every 15 minutes after testing) |
|
Glucagon 1 mg injection (if patient unconscious) |
1 injection in either arm, thigh or buttock (every 15 minutes after testing) |
|
Prevention |
|
|
A1C |
Keep at 7% or lower (most patients 8.5% or less for patients with multiple comorbidities |
|
Insulin |
Rapid-acting insulin such as Humalog (instead of regular) Long-acting basal insulin such as Lantus & Levemir (instead of NPH) |
|
Sulfonylurea |
Gliclazide or glimepiride (instead of glyburide) |
|
Combo |
Metformin + gliptin (Januvia) Metformin + GLP-1 agonist (Victoza) Metformin + pioglitazone (Actos) (Avoid sulfonylurea + insulin) |
DRUG TREATMENTS FOR FIBROMYALGIA

|
Symptom |
Drug |
Comment |
|
Pain |
Acetaminophen (e.g. Tylenol) |
First line |
|
NSAID (e.g. Advil) |
First line (if also other condition such as osteoarthritis) |
|
|
Moderate to severe pain |
Tramadol |
Shown to improve pain & quality of life |
|
Codeine |
|
|
|
Pain, depression, sleep disturbance |
Amitriptyline |
Best choice Start 10mg at bedtime Could go to 25mg at bedtime Side effects: drowsiness, dry mouth, weight gain) |
|
Duloxetine |
Start 30mg – 60mg once daily Higher doses may not be better Response within 8 weeks Side effects: nausea, dry mouth, headache |
|
|
Pain, sleep disturbance |
Pregabalin |
Start 75mg BID Increase to 150mg BID after week Side effects: drowsiness, edema, weight gain |
|
Gabapentin |
Start 300mg at bedtime x 1 week Increase by 300mg/week to 1200-2400mg daily (divided TID) |
|
|
Sleep disturbance |
Nabilone |
Works as well as Amitriptyline for sleep disturbance but more side effects |
BETA BLOCKERS IN CORONARY ARTERY DISEASE

|
Coronary Artery Disease |
Heart Attack |
Systolic Heart Failure |
Beta Blocker Recommendation |
|
Yes |
No |
No |
Only if angina present |
|
Yes |
Yes |
No |
Use 2-3 years; longer if tolerated |
|
Yes |
Yes |
Yes |
Use indefinitely; carvedilol or bisoprolol preferred as they improve survival |
OPIOID DOSING FOR EQUIVALENT ANALGESIA

|
Drug |
Dosage |
|
Morphine (Immediate release) |
10mg q4h |
|
Morphine (Controlled release – e.g. MS Contin, Kadian) |
30mg q12h |
|
Hydromorphone (Immediate release – e.g. Dilaudid) |
2 – 4mg q4h |
|
Hydromorphone (Controlled release – e.g. Hydromorph Contin) |
6mg q12h |
|
Hydromorphone (Extended release – e.g. Jurnista) |
12mg q24h |
|
Oxycodone (Immediate release – e.g. Oxy IR, Percocet) |
5 – 10mg q4h |
|
Oxycodone (Controlled release – e.g. Oxy Contin) |
20 – 30mg q12h |
|
Oxymorphone (Immediate release – e.g. Opana) |
5mg q6h |
|
Oxymorphone (Extended release – e.g. Opana ER) |
10mg q12h |
|
Hydrocodone (Immediate release – e.g. Vicodin) |
10 – 15mg q4h |
|
Codeine |
60mg q4h |
|
Methadone |
Variable – patient tolerance, short-term vs chronic dosing; approx. 7.5mg/24h |
|
Meperidine |
50mg q4h (Acute use only: <48 hrs ) |
|
Fentanyl patches |
25 µg/hr |
EZETROL - EZETIMIBE

Ezetrol – ezetimibe
Cholesterol absorption inhibitor
10MG Tablet
|
Ezetrol alone |
+ statin |
+ fenofibrate |
|
Indicated for 1. the reduction of (total-C), (LDL-C), (Apo B), and (TG) and 2. to increase (HDL-C) in patients with primary (heterozygous familial and non-familial) hypercholesterolemia. |
Same for Ezetrol alone |
Indicated for 1. the reduction of elevated total-C, LDL-C, Apo B, and non-HDL-C in patients with mixed hyperlipidemia. |
Recommended Dose and Dosage Adjustment
· The recommended dose of EZETROL is 10 mg once daily orally, alone, with a statin, or with fenofibrate. EZETROL can be taken with or without food at any time of the day but preferably at the same time each day.
|
Myopathy/Rhabdomyolysis |
Myalgia |
|
Myopathy and rhabdomyolysis are known adverse effects of statins and fibrates. They may also happen to patients on Ezetrol. If a patient complains about muscle pain, consideration given to discontinuation of the drugs. Most cases of myopathy/rhabdomyolysis resolved when drugs were discontinued. |
In controlled clinical trials, the incidence of myalgia was 5.0% for EZETROL vs 4.6% for placebo. Patients should be instructed to contact their physician if they experience persistent and severe muscle pains with no obvious cause. |
Adverse Drug Reaction Overview
· The most commonly reported adverse events in clinical studies were upper respiratory tract infection, headache, myalgia and back pain.
· Concomitant cholestyramine administration decreased the mean AUC of total ezetimibe (ezetimibe+ezetimibe-glucuronide) approximately 55%. EZETROL should be administered either 2 hours or longer before or 4 hours or longer after administration of a bile acid sequestrant.
EZETROL is in a new class of lipid-lowering compounds that selectively inhibit the intestinal absorption of cholesterol and related plant sterols. The molecular target of ezetimibe is the sterol transporter, Niemann-Pick C1-Like 1 (NPC1L1), which is responsible for the intestinal uptake of cholesterol and phytosterols.
Although ezetimibe is rapidly absorbed and is extensively metabolized to an active phenolic glucuronide which reaches the systemic circulation after oral administration, its action is localized at the brush border of the small intestine where it inhibits the absorption of cholesterol, leading to a decrease in the delivery of intestinal cholesterol to the liver. This results in a reduction of hepatic cholesterol stores and an increase in clearance of cholesterol from the blood.
Ezetimibe inhibited the absorption of [14C]-cholesterol with no effect on the absorption of triglycerides, fatty acids, bile acids, progesterone, ethinyl estradiol, or the fat soluble vitamins A and D.
vFEND

|
|
Loading Dose |
Maintenance |
||
|
>40KG |
<40KG |
>40KG |
<40KG |
|
|
Aspergillosis |
400mg q12h |
200mg q12h |
200mg BID |
100mg BID |
|
Candidemia/Candidiasis |
As above |
As above |
As above |
As above |
Treatment duration depends upon the patient's
clinical and mycological response. Patients with candidemia should be treated
for at least 14 days following resolution of symptoms or following last
positive culture, whichever is longer.
Administration
· VFEND (voriconazole) Tablets should be taken at least one hour before, or two hours following, a meal.
· Voriconazole is rapidly and almost completely absorbed following oral administration, with maximum plasma concentrations (Cmax) achieved 1-2 hours after dosing.
· VFEND (voriconazole) is a triazole antifungal agent. The primary mode of action of voriconazole is the inhibition of fungal cytochrome P450-mediated 14α-sterol demethylation, an essential step in ergosterol biosynthesis. The subsequent loss of normal sterols correlates with the accumulation of 14α-methyl sterols in fungi and may be responsible for its fungistatic/fungicidal activity.
QT Interval Prolongation
VFEND (voriconazole) has been associated with prolongation of the QT interval of the electrocardiogram in some patients. Prolongation of QT interval may increase the risk of arrhythmia, cardiac arrests and sudden deaths.
· Due to limited clinical experience, voriconazole should be administered with caution to patients with potentially proarrhythmic conditions such as hypokalemia, clinically significant bradycardia, acute myocardial ischemia, congestive heart failure or congenital prolongation of QT.
· Caution should be exercised if voriconazole is used in patients taking other drugs that may prolong the QT interval, such as antipsychotics, tricyclic antidepressants, erythromycin, Class IA (e.g. procainamide, quinidine) Class III (e.g. amiodarone, sotalol) antiarrythmic agents.
· Drugs metabolized by the hepatic cytochrome P450 isoenzymes CYP2C19, CYP2C9 and CYP3A4 may also affect, or be affected by, voriconazole levels, with possible resulting QT effects. Such drugs include tacrolimus, HIV protease inhibitors, and macrolide antibiotics.
Ophthalmologic
· Voriconazole may cause visual symptoms including photophobia altered/enhanced visual perception, blurred vision and/or color vision change. The majority of visual symptoms appeared to spontaneously resolve within 60 minutes. The effect of VFEND (voriconazole) on visual function is not known if treatment continues beyond 28 days. If treatment continues beyond 28 days, visual function including visual acuity, visual field and color perception should be monitored.
Effects on Ability to Drive and Operating Machines
· Voriconazole may cause visual symptoms including blurring and/or photophobia. The majority of visual symptoms appeared to spontaneously resolve within 60 minutes.
Hepatic
· In clinical trials, there have been uncommon cases of serious hepatic reactions during treatment with VFEND (including clinical hepatitis, cholestasis, and fulminant hepatic failure, including fatalities).. Liver dysfunction has usually been reversible on discontinuation of therapy.
· Acute renal failure has been observed in severely ill patients undergoing treatment with voriconazole. Patients being treated with voriconazole are likely to be treated concomitantly with nephrotoxic medications and have concurrent conditions that may result in decreased renal function.
Skin
There have been cases of exfoliative cutaneous reactions, such as Stevens-Johnson Syndrome (uncommon), toxic epidermal necrolysis (rare) and erythema multiforme (rare) during treatment with voriconazole. Stevens-Johnson Syndrome and toxic epidermal necrolysis should be considered as a differential diagnosis if patients develop prodromal flu-like symptoms (fever, malaise, rhinitis, chest pain. vomiting, sore throat, cough, diarrhea, headache, myalgia and arthralgia). Patients should be closely monitored at the first appearance of a skin rash and voriconazole should be discontinued if lesions progress. Photosensitivity reactions have been observed. It is recommended that patients avoid strong sunlight.
Adverse Drug Reaction Overview
The most frequently reported adverse events (all causalities) in the therapeutic trials were visual disturbances, fever, rash, vomiting, nausea, diarrhea, headache, sepsis, peripheral edema, abdominal pain, and respiratory disorder. The treatment-related adverse events which most often led to discontinuation of voriconazole therapy were elevated liver function tests, rash, and visual disturbances.
Visual Disturbances
· Voriconazole treatment related visual disturbances are common. In therapeutic trials, approximately 21% of patients experienced altered/enhanced visual perception, blurred vision, color vision change and/or photophobia. The visual disturbances were generally mild and rarely resulted in discontinuation. Visual disturbances may be associated with higher plasma concentrations and/or doses.
· The mechanism of action of the visual disturbance is unknown, although the site of action is most likely to be within the retina. The majority of visual symptoms appeared to spontaneously resolve within 60 minutes.
Dermatological Reactions
· Dermatological reactions were common in the patients treated with voriconazole. The mechanism underlying these dermatologic adverse events remains unknown. In clinical trials, rashes considered related to therapy were reported by 7% (110/1655) of voriconazole-treated patients. The majority of rashes were of mild to moderate severity. Cases of photosensitivity reactions appear to be more likely to occur with long term treatment. Patients have developed serious cutaneous reactions, including Stevens-Johnson syndrome (uncommon), toxic epidermal necrolysis (rare) and erythema multiforme (rare) during treatment with voriconazole. Stevens-Johnson Syndrome and toxic epidermal necrolysis should be considered as a differential diagnosis if patients develop prodromal flu-like symptoms (fever, malaise, rhinitis, chest pain. vomiting, sore throat, cough, diarrhea, headache, myalgia and arthralgia).
· Patients should be closely monitored at the first appearance of a skin rash and voriconazole should be discontinued if lesions progress. It is recommended that patients avoid strong, direct sunlight during voriconazole therapy.
TEGRETOL SAFETY INFORMATION

Safety information has been added to Tegretol product monograph.
Serious and sometimes fatal dermatologic reactions, including Toxic Epidermal Necrolysis (TEN) and Stevens-Johnson Syndrome (SJS), have been reported with Tegretol. Occurrence happens more in Asian population (10x more) and may be due to presence of a gene HLA-B1502.
Anti-epileptic drugs have been known to cause the occasional skin reactions.
Tegretol is indicated for the treatment of epilepsy, as well as trigeminal neuralgia, mania, and bipolar disorders.
NUVARING IMPLICATED IN AORTIC THROMBOSIS

A 21-year-old obese woman who smoked 10 to 20 cigarettes per day experienced an aortic thrombosis 15 months after she started using NuvaRing which contains etonogestrel and ethyl estradiol. Her fibrinogen level was high 6.4 g/L (normal 2-4). She was treated with aortic thrombectomy. An embolus was also removed from her left leg.
She did not have a family history of thrombo-embolic disorder, risk factors such as varicose veins, recent injection/infusion, long-distance travel, prolonged immobilization, surgery or trauma.
CENTRUM REFORMULATED

Centrum Select and Centrum Forte have been re-formulated to reflect the latest advances in nutritional science.
|
|
Stronger |
New addition |
Benefit |
|
Vitamin D |
Yes |
|
|
|
Lutein |
Yes |
Prevents macular degeneration/blindness |
|
|
Vitamin K |
Yes |
|
|
|
Lycopene |
Yes |
|
ABREVA

Cold sores are caused by the virus herpes simplex. Once you have the virus, you have it for life. Outbreaks happen when this virus is activated by lip injury (e.g. dry, chapped lips), sunlight, extreme heat or cold, cold or flu, sleep deprivation, stress, menstruation.
Abreva contains 10% docosanol which strengthens cell membranes to prevent virus entry. It is applied as soon as onset of cold sore (e.g. tingling feeling) 5 times a day. Abreva shortens the outbreak by half from 8 days to 4 and eliminate itchy burning pain in 2 days.
To reduce outbreaks, do the following:
- Use a lip moisturizer to prevent chapping
- Use a lip balm with a sunscreen agent
- Avoid contact with people who have cold or flu symptoms
- Find something relaxing to do if stressed out
- Eat well to boost the immune system
DIOVAN 320MG

Diovan (valsartan) is an angiotension receptor blocker indicated for hypertension, either alone or in combination with a thiazide.
It may also be used to reduce cardiovascular mortality after heart attack.
A new strength, 320mg, is now available which is covered by the Ontario Drug Benefit plan. This new double-strength is powerful in that it reduces systolic blood pressure (SBP) by an extra 20%.
MEZAVANT FOR ULCERATIVE COLITIS

Mezavant tablets whole, taking care not to break the outer coating. The outer coating is designed to remain intact until at least pH 7, normally in the terminal ileum, to protect the active ingredient, mesalamine, and ensure its availability throughout the colon.
The concurrent use of mesalamine with known nephrotoxic agents, including non-steroidal anti-inflammatory drugs (NSAIDs) may increase the risk of renal effects. In patients receiving azathioprine or 6-mercaptopurine, concurrent use of mesalamine can increase the potential for blood disorders, especially leucopenia.
Mezavant is intended for once daily, oral administration. The tablets must be swallowed whole and should be taken with food.
The recommended dose for the induction of remission in patients with mild to moderate ulcerative colitis is two to four 1.2 g tablets to be taken once daily for a total daily dose of 2.4 to 4.8 g.
Mezavant features a Multi Matrix System (MMX) to delay and extend the delivery of 5-ASA so that effective concentrations are available throughout the entire colon for 24 hours.
Most common side effects are flatulence and headache.
YASMIN FOR ACNE

Yasmin is now also indicated for acne vulgaris, in addition to conception control.
It contains 30mcg ethinyl estradiol and 3mg drospirenone.
Drospiernone is a progesting with antimineralocorticoid and antiandrogenic activity.
Yasmin should not be used in patients with hyperkalemia (e.g. renal insufficiency, hepatic dysfunction, adrenal insufficiency, concurrent use of ACE inhibitors and blockers,spironolactone, NSAIDs).
Avoid in patients with thromboembolic disorders, cerebrovascular disorders, myocardial infarction, CAD, ophthalmic vascular disease.
Side effects include nausea, vomiting, spotting.
Cigarette smoking is not recommended, especially for women 35 years and older.
FROVA - FROVATRIPTAN FOR MIGRAINE

Frova is a new entry into the market. It belongs to the same family as Imitrex and Zomig.
It is a 5-HT receptor agonist. It helps to prevent excessive dilation of blood vessels in the head.
Cmax is reached at 2 hours. Food lelays Tmax by 1 hour.
Frova is contraindicated in patients with cardiovascular, cerebrovascular and peripheral vascular syndrome, chest pain, arrhythmia, valvular heart disease, uncontrolled hypertension.
Cases of life-threatening serotonin syndrome have been reported during combined use of selective serotonin reuptake inhibitors (SSRIs)/serotonin norepinephrine reuptake inhibitors (SNRIs) and triptans.
Avoid ergots because of extra long vasospastic effect.
The most frequent adverse effects are dizziness, drowsiness, paresthesia.
Dosage is one 2.5mg tablet, may repeat in 4 hours but not more than 2 tablets per 24 hours.
SEROQUEL XR FOR SCHIZOPHRENIA

Once-daily Seroquel is now available, in strengths 50mg, 200mg, 300mg and 400mg.
|
Day 1 |
Day 2 |
Day 3 |
|
300mg |
600mg |
Up to 800mg |
No discontinuation occurs during dose escalation period.
Improvement is seen at week 6 at 600mg or 800mg doses, including aggression, depression.
Most notable side effects are drowsiness, dizziness, , dry mouth and increase in blood sugar.
Caution in elderly patients with dementia because of increased mortality.
CIALIS ONCE A DAY

With a new lower strength 5mg, Cialis can now be taken once a day to maintain sexual readiness for 24 hours.
|
Once-a-day dosing |
On-demand dosing |
|
5 mg once a day |
20mg 30 minutes before sex |
|
May be taken with or without food |
May be taken with or without food |
|
Good for 24 hours |
Good for 36 hours |
Cialis should be avoided in heart patients, especially those on nitrates.
Side effects include headache, flushing, and indigestion.
Caution in renal and hepatic patients, or those on ritonavir and ketoconazole.
ACLASTA FOR OSTEOPOROSIS

Aclasta (zoledronic acid 5mg/100ml) is a once a year IV infusion for the treatment of osteoporosis in postmenopausal women to reduce the incidence of hip, vertebral and nonvertebral fractures. The infusion takes 15 minutes.
Before administration, the patient must have adequate blood levels of calcium and vitamin D.
Initial side effects include fever, muscle aches, flu-like symptoms.
Aclasta may cause deterioration of kidney function.
Notable drug interaction involves aminoglycosides which result in lower serum calcium.
AMARYL FOR TYPE 2 DIABETES

AMARYL (glimepiride) is a sulfonylurea. 1, 2, 4mg
Mechanism of Action
The primary mechanism of action of glimepiride is stimulating the release of insulin from functioning pancreatic beta cells. In addition, glimepiride administration can lead to increased sensitivity of peripheral tissues to insulin.
After oral administration, glimepiride is completely (100%) absorbed from the GI tract.
Recommended Dose and Dosage Adjustment
Usual Starting Dose: 1 mg once daily, administered with breakfast or the first main meal.
Usual Maintenance Dose: 1 to 4 mg once daily. Maximum: 8 mg once daily.
AMARYL-Metformin Combination Therapy: Combination therapy with AMARYL and metformin may be used in patients who do not respond adequately to the maximal dose of AMARYL or in secondary failure patients.
· Study indicated that the combination of metformin and glimepiride was more effective than either treatment alone, with regards to improving HbA1C, fasting blood glucose and postprandial blood glucose levels.
AMARYL-Insulin Combination Therapy: Combination therapy with AMARYL and insulin may be used in secondary failure patients. The recommended AMARYL dose is 8 mg once daily administered with the first main meal. After starting with low-dose insulin, upward adjustments of insulin can be done approximately weekly as guided by frequent measurements of fasting blood glucose.
Changeover from Other Oral Hypoglycemic Agents: It is recommended that the procedure be the same as for initial dosage starting with daily doses of 1 mg.
Over a period of time, patients may become progressively less responsive to therapy with oral hypoglycemic agents because of deterioration of their diabetic state. This is detected with regular blood glucose monitoring. Options include switching to another sulfonylurea, adding a different antidiabetic, or insulin.
Hypoglycemia
All sulfonylurea drugs are capable of producing severe hypoglycemia. Signs of severe hypoglycemia can include disorientation, loss of consciousness, and seizures. Hypoglycemia is more likely to occur when caloric intake is deficient, after severe or prolonged exercise, when alcohol is ingested, or when other drugs with blood-glucose lowering potential are used.
It has been suggested, based on a study conducted by the University Group Diabetes Program (UGDP), that certain sulfonylurea antidiabetic agents increase cardiovascular mortality in diabetic patients, a population at greater risk of cardiovascular disease.
Alertness and reactions may be impaired due to hypo- or hyperglycemia, especially when beginning or after altering treatment or when AMARYL (glimepiride) is not taken regularly.
Glimepiride is metabolized by cytochrome P450 2C9 (CYP2C9). This should be taken into account when glimepiride is coadministered with inducers (e.g. rifampicin) or inhibitors (e.g. fluconazole) of CYP 2C9.
Both acute and chronic alcohol intake may potentiate or weaken the blood-glucose-lowering action of AMARYL in an unpredictable fashion.
Although ASA increases clearance and decreases blood levels of Amaryl, blood glucose concentrations remain stable.
Some drugs produce hyperglycemia and may lead to loss of glycemic control.
- Thiazides
- Corticosteroids
- Epinephrine and other sympathomimetic agents
- Glucagon
- Estrogen and progestagens
- Thyroids
JANUVIA FOR TYPE 2 DIABETES

Januvia (sitagliptin) is the first DDP-4 inhibitor approved for the treatment of type 2 diabetes in Canada. It works by allowing gastrointestinal hormones GIP and GLP to continue exert their effect (enhancing insulin response and reducing glucagon effect on blood sugar) in response to ingestion of food without being degraded by DDP-4.
It is available in 100mg tablets.
Dosage is one a day, regardless of meal. Absorption is rapid and nearly complete.
It may be used alone or in combination with other drugs (pioglitazone, metformin, glimepiride).
It is not recommended in cases of severe hepatic or renal impairment.
There have not been clinically significant drug interactions.
There have been higher incidences (than placebo) upper respiratory tract infection, nasopharyngitis, and headache.
AVALIDE CAN BE 1ST LINE

Avalide is normally indicated, not 1st line, for the treatment of hypertensive patients with Type 2 diabetes mellitus and renal disease to reduce the rate of progression of nephropathy (increased microalbuminuria and serum creatinine).
However, Avalide has been shown to induce dramatic reduction (as much as 30 mm HG) within a week. It is now indicated for initial therapy for “severe” hypertension (sitting DBP>110mm Hg).
Most common side effects: dizziness, fatigue, headache.
INFANT COUGH & COLD MEDICINE

In response to Health Canada (Public Enquiry 1-866-225-0709) concern, major makers of children medicine are removing their under-2-years-of-age products from the market. These products generally contain more than 1 ingredient and pose life-threatening risks to young children/infants due to overdose. Common cold, a viral infection, usually goes away on its own. The condition can be easily managed by rest, sufficient fluid, and comfort measures. If symptoms persist or deteriorate, bacterial infection (e.g. pneumonia) may be suspected and medical attention is in order
|
Product |
UPC (Barcode) |
|
Benylin Oral Infant Drop 15ml |
06024526135 |
|
Benylin Infant Stuffy Nose 15ml |
06024595465 |
|
Dimetapp Infant Cold & Fever Drop 50ml |
06210722855 |
|
Dimetapp Infant Cold Drop 50ml |
06210723220 |
|
Tylenol Cold Drop 24ml |
06454130037 |
|
Little Colds Decongestant Plus Cough 30ml |
75618412188 |
|
Little Colds Multi-Symptom Cold Formula 24ml |
75618412199 |
|
Triaminic Toddler Cough and Cold Mixed Berry 16s |
05847810164 |
Johnson & Johnson (maker of Tylenol and Benylin) accepts consumer return (Consumer Hotline 1-800-265-7323)
CAMPRAL (ACAMPROSATE) FOR ALCOHOL DEPENDENCE

Campral prevents relapse in patients who have quit drinking. Management of alcohol dependence is challenging because half of those will go back to drinking after 2 years abstinence.
Chronic exposure to alcohol results in dysregulation of neuronal excitation and inhibition which are mediated by glutamate and GABA. Campral act on these receptors to restore the normal balance between gluatmatergic excitation and GABAergic inhibition.
Campral tablets are enteric-coated. Each tablet contains 333mg acamprosate. There are 7 strips of 12 tablets each in a box.
Intial dosage is 1 tablet TID. Increase by 1 tablet a week until average dosage of 2 tablets TID is reached.
Campral is well tolerated. Most common side effects are nausea, upset stomach, and diarrhea. Drug interaction potentials are low.
ALVESCO (CICLESONIDE) FOR ASTHMA PREVENTION
Avlesco is used once daily for the prevention of asthma in patients 18 years and older. Starting dose (regardless of previous use of inhaled corticosteroid or bronchodilator) is 400 mcg once daily. Optimum range is 100 to 800 mcg per day. If 800mcg is used, it should be divided into 400mcg BID. The main ingredient (ciclesonide) inside the canister is in solution form so no shaking is necessary. Each canister contains 120 doses (100mcg or 200mcg) and should be primed, when new, by wasting 3 sprays. Once inhaled, ciclesonide is converted by esterases in the lungs to its active metabolite, 21 des-methylpropionyl-ciclesonide (M1), which is a potent glucocorticoid that binds to glucocorticoid receptors in the lung resulting in local pronounced anti-inflammatory activity.
If Alvesco is to be discontinued, it should be done gradually. Patients who are on drugs that suppress the immune system are more susceptible to infections than healthy individuals. Therapeutic dosages of inhaled corticosteroids may cause the appearance of C. albicans (thrush) in the mouth and throat. Patients may find it helpful to rinse and gargle with water after using Alvesco. Systemic effects of inhaled corticosteroids may occur, particularly at high doses prescribed for prolonged periods. These effects are much less likely to occur than with oral corticosteroids. Possible systemic effects include adrenal suppression, growth retardation in children and adolescents, decrease in bone mineral density, cataract and increased intraocular pressure, with or without glaucoma. Therefore, it is important that the dose of inhaled corticosteroid is titrated to the lowest dose at which effective control of asthma is maintained.
SATIVEX SPRAY NOW FOR CANCER PAIN AS WELL

Sativex buccal spray was previously indicated solely for neuropathic pain in multiple sclerosis. In August 2007, it received approval for use in cancer patients who, despite highest dose opioid therapy, still experience moderate to severe pain. On average, cancer pain patients require 8 sprays per day (compared to 5 sprays for MS). Each spray delivers 2.7mg THC (tetrahydrocannabinol) and 2.5mg CBD (cannabidiol) which are thought to act via cannabinoid receptors throughout the CNS. THC is a psychotropic agent which may produce physical and psychological dependence, as well as potential for abuse.
Patients should be screened for heart disease, hypertension, and psychotic disorder.
Patient must be 18 and over.
Application site reactions include stinging and ulceration (rotate site is recommended).
CNS reactions include dizziness, drowsiness, disorientation, memory impairment, confusion.
Psychiatric symptoms include anxiety, illusion, delusion, disorientation, hallucination, paranoia.
Cardiovascular effects include higher pulse rate, hypotension, and fainting episodes.
THC may raise seizure threshold.
HEALTH CANADA WARNINGS AND ADVISORIES

Consumers are advised not to use the following products.
|
Product |
Reason |
|
MdMt |
Contains methyl-1-testosterone & methyldienolone |
|
Jie Jie Pills & Chuan Xiong Cha Tiao Wan |
Contain aristoiochic acid which may cause kidney failure and cancer |
|
Darling Capsules, Dafi Capsules, Spanish Fly Capsules |
Contains sildenafil |
|
Dai Dai Hue Jiao Nang |
Contain sibutramine |
|
Kui Hua Chut Lee San Bird’s Nest & Pearl |
Contain bacteria |
|
Optimum Health Care Sleep Easy |
Contain clonazepam |
|
Zencore Tabs & Liviro-3 |
Contain analog of tadalafil |
|
Neem Active Toothpaste with Calcium |
Contain toxic diethylene glycol |
|
Resolve Smoke Cessation Aid |
May cause kidney, liver or red blood cell damage |
CHAMPIX DOSING INFORMATION
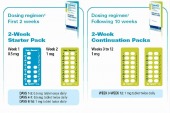
Champix is available in Starter Pack and Continuation Pack. Both packs cover a 2-week period.
In the Starter Pack, the first week is a blue strip with 0.5mg tablets only. The second week is a greenish strip with 1mg tablets only.
The Continuation Pack is made up of 2 strips of all 1mg tablets.
For the first week, the patient takes 1 tablet daily from the blue strip (i.e. 0.5mg) for the first 3 days. Then from day 4 to 7 of the first week, the patient takes 1 tablet twice daily (all 0.5mg tablets). The patient continues to smoke for the first week
For the second week on, the patient increased the dosage to 1 tablet twice daily (all tablets now 1mg).
Also, as of the first day of the second week (or the 8th day from the beginning of treatment), the patient stops smoking.
The course of therapy is for 12 weeks. If the patient is able to quit in 12 weeks but worried about relapse, he/she may use Champix for another 12 weeks to ensure “stay quit”.
CHAMPIX - VARENICLINE FOR SMOKE CESSATION

Pfizer Canada just introduced CHAMPIX into the market, an innovative smoking cessation therapy to help motivated people quit smoking and stay quit. It is not an antidepressant and its chemical structure is different from Zyban - bupropion (C13H13N3.C4H6O6 and C13H18CINO respectively). Its partial agonist activity at brain nicotine receptor stimulates release of a little dopamine to ease craving but mostly, it blocks receptor sites to prevent nicotine from cigarettes to exert addictive effects.
Champix provides twice the odds of quitting smoking when compared against bupropion.
The initial course of Champix therapy is 12 weeks. An additional 12 weeks may be considered to help successful candidates to stay quit.
Side effects may appear in initial weeks and include nausea, headache, constipation, gas, trouble sleeping, and abnormal dreams.
Champix tablets may be taken with or without food.
When on Champix, nicotine patches and gums are not recommended to be used concurrently.
PARIET 10MG SUPPLY PROBLEM
Janssen-Ortho announced that Pariet 10mg will not be available for 3 to 4 months.
Meanwhile, 20mg strength is freely available.
DRUG RECALL: ZELNORM (TEGASEROD)
NOVARTIS, maker of Zelnorm (tegaserod) for irritable bowel syndrome and chronic constipation, has decided to stop its marketing and sales in Canada. Clinical studies indicated a statistically significant increase in the incidence of cardiovascular ischemic events (strokes and heart attacks), especially among those with risk factors of high blood pressure, high cholesterol, previous cardiovascular disease. Patients should stop using Zelnorm and contact their doctors for options. There are no known risks for abrupt discontinuation of Zelnorm. You should return any unused Zelnorm tablets to your pharmacy where your prescription was filled where you will be reimbursed the amount that you have paid.
BONE FRACTURES FROM AVANDIA USE

Analyzing data from ADOPT Study in Type 2 diabetes show women on rosiglitazone (Avandia) experienced more fractures in their upper arm, hands and feet. This is different from osteoporosis fractures which occur in hips and spine. Men do not suffer the same side effect. It is recommended women users of Avandia be more vigilant on their bone health.
DIOVAN 320MG
DIOVAN 320mg is now available as a one a day tablet, doubling the previous highest strength of 160mg. It is found that by doubling the daily dose from 160mg to 320mg reduces the Systolic BP by additional 20% which improves survival. Diovan is the first ARB approved for both reducing BP and CV mortality in cases where patients are suffering from both left ventricular dysfunction (LVD) and acute myocardial infarction when ACEI is inappropriate. Diovan can be used in patients with mild to moderate liver diseases and renal impairment.
ARANESP (DARBEPOETIN)

Aranesp (darbepoetin) is an erythropoiesis regulating hormone made up of a protein produced in ovary cells using recombinant DNA technology. It is used in treatment of anemia caused by either chronic renal failure (CRF) or by chemotherapy in cancer patients. Note: Ontario Drug Benefit only covers chemotherapy-induced anemia. Patients with uncontrolled hypertension should not receive Aranesp. Aranesp may increase risk of thrombotic vascular events which can be fatal. Use with caution in patients with history of seizures. Seizures have occurred during Aranesp treatment among these patients. It is not known if seizures are caused by rise in blood pressure or hemoglobin. Long-term treatment may lead to Pure Red Cell Aplasia (PRCA), especially in CRF patients. Hemoglobin level must not be allowed to rise beyond 120g/L. Iron supplement is recommended for patients whose ferritin level is below 100. Aranesp is administered either IV or SC as a single injection administered weekly or once every two weeks. The dose should be started and slowly adjusted. The recommended starting dose of Aranesp for the correction of anemia in CRF patients is 0.45 µg/kg body weight, administered as a single IV or SC injection once weekly. Aranesp dosage should be adjusted to maintain a target hemoglobin not to exceed 120 g/L. Aranesp comes in single-dose prefilled syringes and single-dose vials. Prefilled syringes come in strengths 10, 15, 20, 30, 40, 50, 60, 80, 100, 130, 150, 200, 250, 300, 400, 500 µg. Store at 2 to 8°C. Do not freeze or shake. Protect from light.
PAXIL IN PREGNANCY
Paxil is not recommended for women during or just before pregnancy. Two studies have shown pregnant women who were on Paxil during their first trimester had twice the incidence of giving birth to babies with heart defects.
CHOCOLATE BAR RECALL

Hershey canada issued voluntary recall of some of their products, e.g. Oh Henry chocolate bars because of Salmonella contamination. These affected products were made in Smith Falls between October 15 and November 10, 2006. All Hershey products in our chain of IDA stores are not affected by this recall. Thank you for your patronage.
HEALTH CANADA ADVISORY ON GLEEVEC (IMATINIB)

Ten patients receiving Gleevec (Imatinib), a tyrosine kinase inhibitor, for chronic myeloid leukemia CML) or gastrointestinal stromal tumors (GIST), developed significant left ventricular ejection fraction reduction and congestive heart failure (CHF). It is hypothesized that Gleevec inhibits heart cell kinase resulting in death of some heart cells. It is recommended that ? Any patients using GLEEVEC*, with known cardiac disease or risk factors for cardiac failure, should be monitored carefully. ? Any patient using GLEEVEC* who has symptoms or signs suggestive of Congestive Heart Failure (i.e., edema, dyspnea, pleural effusion or pericardial effusion) should receive timely and thorough evaluation and treatment. ? In patients with known underlying heart disease or in elderly patients, a baseline evaluation of Left Ventricular Ejection Fraction is recommended prior to initiation of GLEEVEC* therapy.
CLOBEX FOR PSORIASIS

Galderma sent out notices to pharmacies championing its Clobex Lotion as the only logical choice for patients suffering from either plaque psoriasis or atopic dermatitis because it is the only lotion on the market that is free from irritating fragrance, preservatives, and alcohol. The lotion format penetrates the skin better than cream. Its non-alcohol base is moisturizing. It contains the powerful cortisone Clobetasol 0.05%.
AROMASIN FOR BREAST CANCER

Aromasin (exemestane) is an aromatase inactivator (AI). It disables aromatase enzyme which is required to produce estrogen in post-menopausal women. Target patient is in early stage of breast cancer, age post menopause, estrogen-receptor positive, and has undergone tamoxifen therapy for at least 2 years. Dosage is one tablet (25mg) once daily with a meal. Side effects include hot flushes, diarrhea, alopecia, ischemic cardiac events, hypercholesterolemia, osteoporosis.
SINGULAIR NEW USE
Since launched in 1998, Singulair has always been indicated for prophylaxis and chronic treatment of asthma in patients from 2 years and up. Now Merck Frosst announced Singulair is also indicated for the relief of symptoms of seasonal allergic rhinitis (hay fever) in patients 15 years and older. There are no noticeable side effects when used for hay fever but in asthma patients, there is a little over 1% incidence of abdominal pain and headache. Patients with mild to moderate hepatic insufficiency may produce a higher blood level and longer elimination of the drug. Elevations in serum transaminases have been observed but mostly transient although jaundice has been reported.
SUTENT FOR KIDNEY CANCER

Sutent (sunitinib made by Pfizer) is now approved by Health Canada for treatment of metastatic renal cell carcinoma (MRCC) and GI stromal tumour (GIST). It comes in 12.5mg, 25mg, and 50mg capsules. Standard dosage is 50mg once daily for 4 weeks followed by 2 weeks off. Sutent inhibits receptor tyrosine kinases (RTK) which are implicated in tumour growth. Adverse effects include fatigue, nausea, diarrhea, skin discoloration. Avoid concomitant administration with CYP3A4 inhibitors and QT-interval prolonging drugs. Pfizer has a First Resource Program in place to provide free Sutent therapy to eligible patients (1-888-963-4778).
LAMICTAL SAFETY ALERT

Pregnant women exposed to Lamictal (lamotrigine) in the first trimester may give birth to infants with cleft lip and cleft palate. The rate is 1 in 100 births (compared to 0.03 per 100 in non-Lamictal population). To facilitate monitoring fetal outcomes of pregnant women exposed to lamotrigine, patients are encouraged to enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry by calling (888) 233-2334.
FLOMAX TO BE REPLACED BY CR
Boehringer has decided to discontinue Flomax in favor of Flomax CR. The new CR version has advantages over the regular version: ? Can be taken with or without food ? Blood levels are smooth & free from spikes ? Less dizziness ? Less orthostatic hypotension ? 38% cheaper
REMICADE SAFETY ALERT
REMICADE® (infliximab) is a Biological Response Modifier that is directed against the cytokine tumour necrosis factor-α(TNFα). It is indicated for the treatment of adults with rheumatoid arthritis, ankylosing spondylitis, Crohn's disease, ulcerative colitis, psoriatic arthritis, and chronic plaque psoriasis. Six (6) post-marketing cases of a rare type of lymphoma called hepatosplenic T-cell lymphoma (HSTCL) have been reported in pediatric and young adult patients taking REMICADE® for Crohn's disease. Five of the six cases (all U.S.) resulted in death. All cases reported concomitant or past use of other immunosuppressive agents, including azathioprine or 6-mercaptopurine. Although lymphomas have been reported in Canada in patients receiving REMICADE® for Crohn?s disease and other indications, there have been no reports of HSTCL. REMICADE® is not authorized for pediatric use in Canada.
APTIVUS SAFETY ALERT

Health Canada issued in July, 2006, a safety alert on Aptivus antiviral agent. APTIVUS co-administered with low dose ritonavir is indicated for combination antiretroviral treatment of HIV-1 infected adult patients. There have been 14 reports of intracranial hemorrhage (ICH), including 8 fatalities, in 6840 HIV-1 infected patients receiving APTIVUS in clinical trials. Many of the patients experiencing ICH in the APTIVUS clinical trials had other medical conditions (CNS lesions, head trauma, neurosurgery, coagulopathy, hypertension or alcohol abuse) or were receiving concomitant medications, including anticoagulants and antiplatelet agents, that may have caused or contributed to these events. Tipranavir has been observed to inhibit human platelet aggregation in vitro. APTIVUS/ritonavir should therefore be used with caution in patients who may be at risk for increased bleeding from trauma, surgery or other medical conditions, or who are receiving medications known to increase the risk of bleeding, including antiplatelet agents or anticoagulants.
TWINJECT EXPIRY DATE
The expiry date that shows on Twinject is in the format of Month/Day/Year but expressed in numerals only which creates confusion as to the exact expiry date because one could interpret Day/Month/Year. The maker, Paladin, promised to have the situation rectified by printing the month in letters instead of numbers in future productions.
NEW LABELLING REQUIREMENT FOR BLACK COHOSH

This is an Australian initiative only. But since black cohosh is widely used in Canada and United States for relief of menopause symptoms, consumers must pay heed to warning. "Warning: Black Cohosh may harm the liver in some individuals. Use under the supervision of a healthcare professional". There were 47 cases of liver reactions worldwide. In Australia, 4 patients were hospitalised, including 2 who required liver transplantation. There is sufficient evidence of a casual assocation hbetween Black Cohosh and serious hepatitis. However, considering the widespread use of Black Cohosh, the incidence of liver reaction appears to be very low.
TRIAMINIC VAPOUR PATCH RECALL

Novartis Consumer Products is issuing a Recall Notice on all its Triaminic Vapour Patch Products on the advice of Health Canada. The problem is not with the product itself when used properly. It is due to the accidental poisoning when children inadvertently ingest the patch when they are not supposed to swallow (it is an inhalation product).
EVISTA STROKE RISK

Eli Lilly Canada, maker of Evista, released a statement regarding increased mortality rate due to stroke, albert just slightly, in postmenopausal women on Evista. Evista is a popular and effective drug to prevent osteoporosis in menopausal women. Women enrolled in the RUTH trial had either documented coronary heart disease, lower extremity arterial disease, hypertension, diabetes mellitus, hyperlipidemia, tobacco smoking, or age 70 and over. Women with a history of stroke or stroke risk factors such as transient ischemic attack or atrial fibrillation should be carefully assessed before put on Evista.
L-Arginine Recall

Swiss Herbal Remedies Ltd. issued a recall notice to pharmacies to remove from shelf of all L-Arginine 500mg Lots 3670 and 4168 Prodocut Code 171101 and UPC Code 68120711015. This follows recommendation from Natural Health Products Directorate prohibiting "exercise and diuretic claims" involving L-Arginine. And patients with recent myocardial infarction (heart attack) should not consume L-Arginine.
ReNu Contact Lens Solution

Bausch & Lomb Canada issued a clarifying statement regarding its ReNu MultiPlus Conctact Lens Solution currently on the Canadian market. This solution contains a totally different disinfectant from the one which was recently pulled from the American market under the name ReNu with MoistureLoc which allegedly caused a number of cases of Fusarium keratitis among users. The Canadian product is totally safe. Its main disinfectant is polyhexamethyl biguanide which is the same chemical in 2 other competing brands Complete & SoloCare. ReNu MultiPlus also contains a borate buffer to synergistically increase disinfecting efficacy.
SureStep Blood Glucose Meter Recall

Lifescan is recalling its SureStep Blood Glucose Meters that bear Serial Numbers in the range from L3152Rx xxxxx to L5348Rx xxxxx. If you own a meter that falls in the above range, please call 1-800-663-5521. Lifescan will send you a replacement. The faulty meters have a malfunctioned LCD display that don't let you read properly.
Tequin messes up blood sugar
A Canadian study showed Tequin (gatifloxacin), one of popular fluoroquinolone antibiotics, caused 17 times more blood sugar abnormalities which are potentially life-threatening.
Symptoms of low blood sugar include sweating, shakiness, confusion, light-headedness, a racing heart beat and trouble speaking. As the levels drop further, a person may experience seizures, coma and eventually death. The hallmarks of high blood sugar include excessive urination and thirst, confusion, and nausea. The condition can escalate to the point of coma and death. Diabetic patients should not take Tequin.
New safety information regarding Paxil (paroxetine
An U.S. epidemiologic study and a Swedish study both find a 1.5 to 2-fold increase in cardiac malformations in infants exposed to paroxetine (as compared to other antidepressants). Maker of Paxil, GlaxoSmithKline, now recommends that if a patient becomes pregnant while taking paroxetine, she should be informed of the increased risk to the fetus. Consideration should be given to switching to other treatment options, including another antidepressant or non-pharmaceutical treatment such as cognitive behavioural therapy.
Pseudoephedrine and Ephedrine to move behind the c

Effective April 10th, 2006, single-entity products containing either pseudoephedrine or ephedrine will be moved from Schedule III to Schedule II on the list of drugs for sale in Ontario. The end result is that these products will have to be asked for at a pharmacy behind the counter. This change is implemented to address the problem of clandestine production of methamphetamine. By placing these products behind-the-counter, it makes difficult for producers of crystal meth to access the precursor ingredients required to make it.
